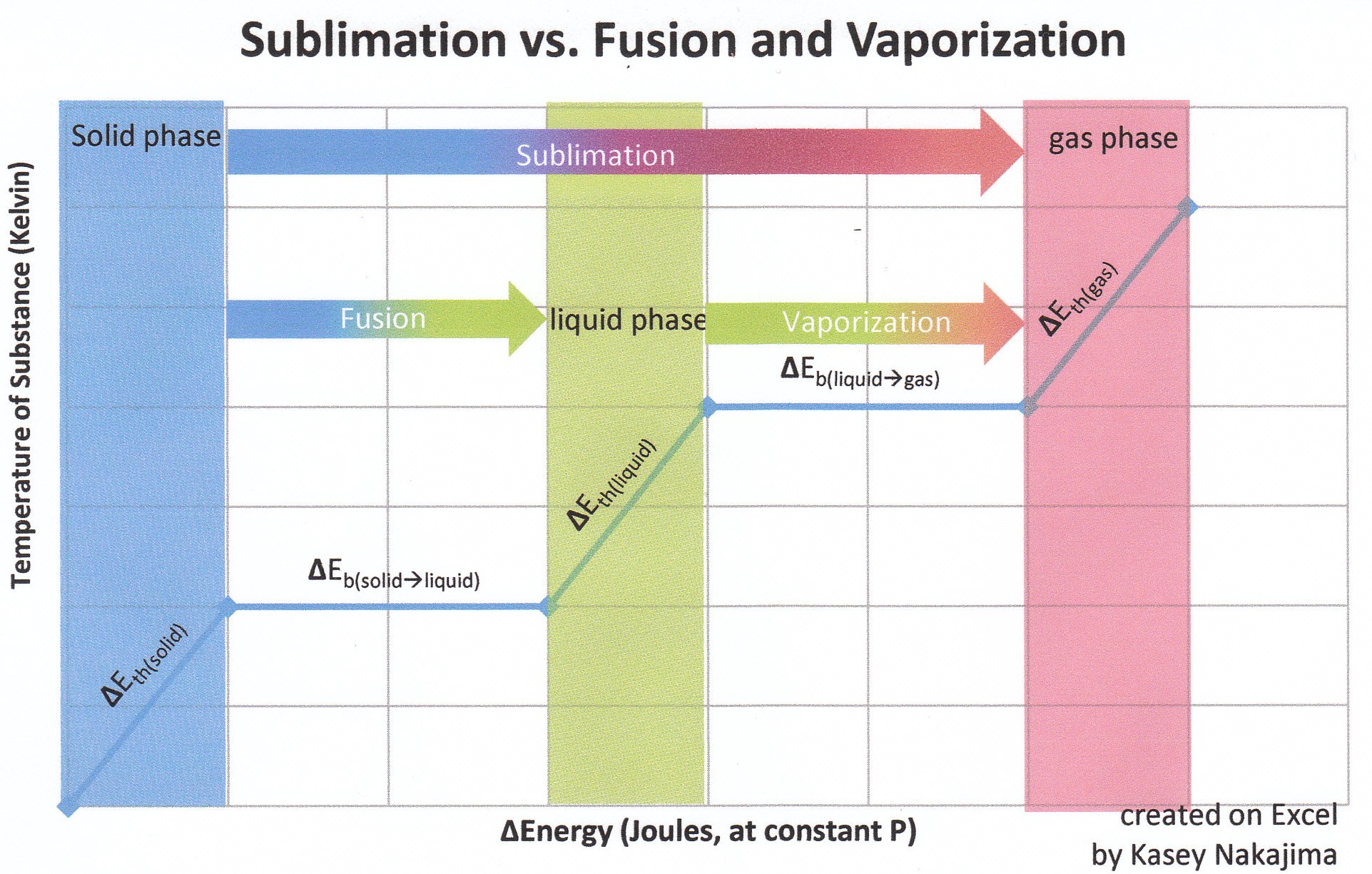
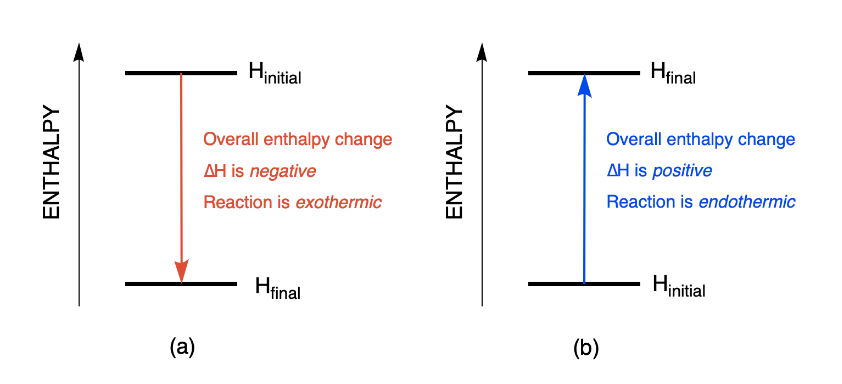
Calculations in Chapter 10. Molar Enthalpy of Fusion Used when melting or freezing = ___energy ____ mol of substance Can be arranged to find any of the. - ppt download

Calculations in Chapter 10. Molar Enthalpy of Fusion Used when melting or freezing = ___energy ____ mol of substance Can be arranged to find any of the. - ppt download
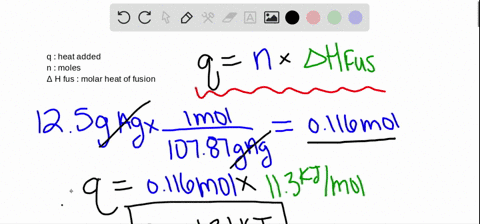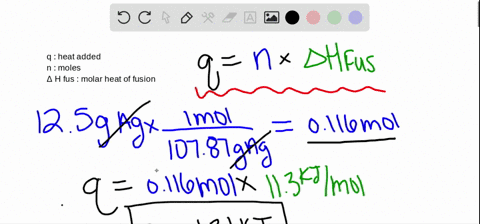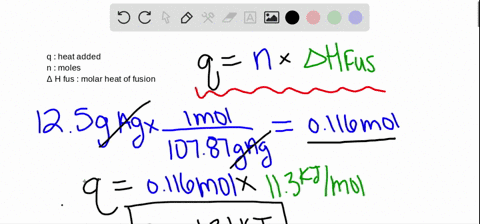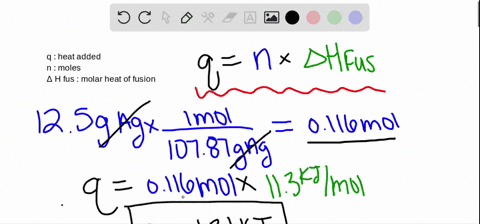
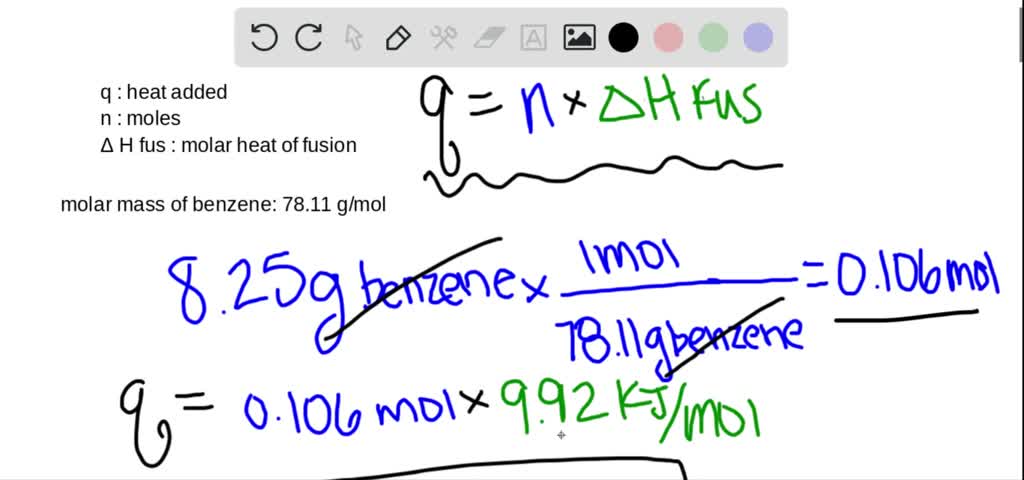
SOLVED:The molar heat of fusion of benzene is 9.92 kJ / mol . Its molar heat of vaporization is 30.7 kJ / mol . Calculate the heat required to melt 8.25 g





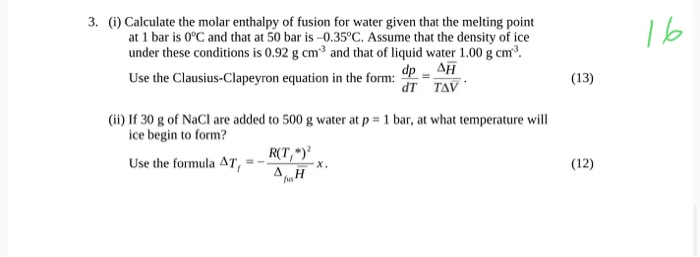

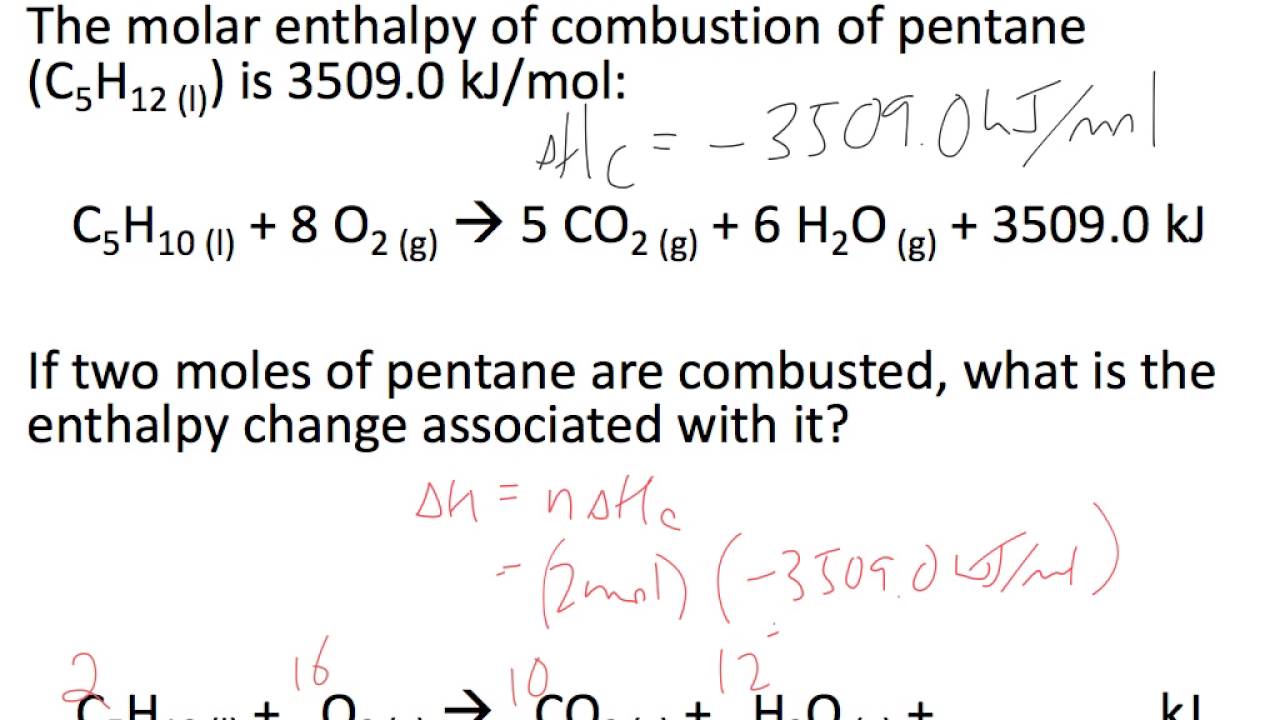







:max_bytes(150000):strip_icc()/GettyImages-914268080-0538c57bcc304e49be217291114f221a.jpg)